
Factor VIII levels at 44%, 4 days after infusion
Hypothetical patient and scenario
Factor VIII levels at 44%, 4 days after infusion
Hypothetical patient and scenario
Ready for a once-a-week infusion with proven bleed protection? Get to know ALTUVIIIO®!
ALTUVIIIO is a first-in-class, Factor VIII replacement therapy that keeps factor activity levels higher for most of the week with one weekly infusion

The only once-weekly prophylaxis factor infusion
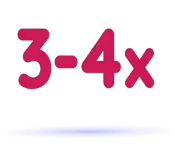
3-4x longer half-life, in a separate study with EHL and SHL therapies*
In a Phase 1 study, ALTUVIIIO had a 3-4x longer half-life than EHL and SHL therapies

*
This is information from a study of 13 previously treated adults with severe hemophilia A, that had the goal of comparing how long ALTUVIIIO, Adynovate® [Antihemophilic Factor (Recombinant), PEGylated], and Advate® [Antihemophilic Factor (Recombinant)] stayed in the body after 1 dose. Half-life was 43 hours for ALTUVIIIO, 15 hours for Adynovate, and 11 hours for Advate.
Adynovate and Advate are registered trademarks of Baxalta Incorporated, a Takeda company.
EHL=extended half-life; SHL=standard half-life.
Adynovate and Advate are registered trademarks of Baxalta Incorporated, a Takeda company.
EHL=extended half-life; SHL=standard half-life.
In the XTEND-1 study, average Factor VIII levels stayed above:
40%
For most of the week in adults
(near-normal to normal range)
(near-normal to normal range)
18%
On average for adults, for the entire week
Average trough levels were 9% for adolescents aged 12 years to under 18 years.
Average trough levels were 9% for adolescents aged 12 years to under 18 years.
How was ALTUVIIIO studied?
ALTUVIIIO was studied in XTEND-1 for 1 year
159
=
Adults and Adolescents
158 males and 1 female with severe hemophilia (aged 12 years and older)
158 males and 1 female with severe hemophilia (aged 12 years and older)
133
+
People in group 1 switched to ALTUVIIIO prophylaxis from prior prophylaxis therapy.
Efficacy of prophylaxis was evaluated in 128 of these patients.
Efficacy of prophylaxis was evaluated in 128 of these patients.
Included 1 female participant
26
People in Group 2 switched from prior on‑demand therapy to ALTUVIIIO on‑demand for 26 weeks, and finally to ALTUVIIIO prophylaxis for another 26 weeks
159
Adults and Adolescents
158 males and 1 female with severe hemophilia (aged 12 years and older)
158 males and 1 female with severe hemophilia (aged 12 years and older)
=
133
People in group 1 switched to ALTUVIIIO prophylaxis from prior prophylaxis therapy.
Efficacy of prophylaxis was evaluated in 128 of these patients.
Efficacy of prophylaxis was evaluated in 128 of these patients.
Included 1 female participant
+
26
People in Group 2 switched from prior on‑demand therapy to ALTUVIIIO on‑demand for 26 weeks, and finally to ALTUVIIIO prophylaxis for another 26 weeks
Finding people’s mean annualized bleed rate was the primary goal of the study
Proven Bleed Protection with ALTUVIIIO Prophylaxis
Data from the XTEND-1 study
0
Median bleeds per year*
(median annualized bleed rate)
(median annualized bleed rate)
0.7
Mean bleeds per year*
(mean annualized bleed rate)
PRIMARY OUTCOME
(mean annualized bleed rate)
PRIMARY OUTCOME
0
Median joint bleeds per year*
(median annualized joint bleed rate)
(median annualized joint bleed rate)
How were bleeds and joint bleeds measured in the trials?
- Median ABR was the middle number of all ABRs, when everyone’s ABR was ordered from least to greatest
- Mean ABR was the average number based on everyone’s ABR
- Median ABR was the middle number of all ABRs, when everyone’s ABR was ordered from least to greatest
- Mean ABR was the average number based on everyone’s ABR
Significant improvement in bleed protection with ALTUVIIIO prophylaxis
In Group 1:
133 people aged 12 years and older had prior prophylaxis therapy and switched to ALTUVIIIO. 78 of those people participated in a separate study to measure their ABRs on their prior prophylaxis. Comparing the results of the 78 people who participated in both studies showed:
133 people aged 12 years and older had prior prophylaxis therapy and switched to ALTUVIIIO. 78 of those people participated in a separate study to measure their ABRs on their prior prophylaxis. Comparing the results of the 78 people who participated in both studies showed:
On Average
People went from 3 bleeds to less than 1 bleed a year (mean ABR 0.7). That’s a 77% reduction in yearly bleeds (mean)*
People went from 3 bleeds to less than 1 bleed a year (mean ABR 0.7). That’s a 77% reduction in yearly bleeds (mean)*
And
Over 52 weeks on prophylaxis 64% of people had 0 bleeds* vs 42% on prior prophylaxis
Over 52 weeks on prophylaxis 64% of people had 0 bleeds* vs 42% on prior prophylaxis
*
Data based on treated bleeds.
ABR=annualized bleed rate.
ABR=annualized bleed rate.
Know your joints are protected
Over 52 weeks on ALTUVIIIO prophylaxis
72% of people had zero joint bleeds
72% of people had zero joint bleeds
Joint Health Scores
- A mean change of -1.5 (-2.7, -0.3) from starting point was observed in Hemophilia Joint Health Score (HJHS) total score
- HJHS is a validated tool used to measure joint health function. The study was designed so the investigators knew the patients were taking ALTUVIIIO, which may have impacted their assessment of the patients’ HJHS score

100%
Target joint resolution
Target joint resolution
- Target joints are 3 or more spontaneous bleeds in a major joint in a consecutive 6-month period
- Target joints were considered resolved if 2 or fewer bleeds occurred in a target joint in 12 months
*
Data based on treated bleeds.
Demand fewer yearly bleeds
Switch it up from on-demand to prophylaxis with ALTUVIIIO
In Group 2:
26 people aged 12 years and older were treated on-demand with ALTUVIIIO, then switched to ALTUVIIIO prophylaxis for 26 weeks.
26 people aged 12 years and older were treated on-demand with ALTUVIIIO, then switched to ALTUVIIIO prophylaxis for 26 weeks.
On Average
People who switched from ALTUVIIIO on-demand to ALTUVIIIO prophylaxis went from 21 bleeds to less than 1 bleed a year (mean ABR 0.7). That’s a 97% reduction in yearly bleeds (mean)*
People who switched from ALTUVIIIO on-demand to ALTUVIIIO prophylaxis went from 21 bleeds to less than 1 bleed a year (mean ABR 0.7). That’s a 97% reduction in yearly bleeds (mean)*
And
Over 26 weeks on prophylaxis 77% of people had 0 bleeds* and 81% of people had 0 joint bleeds*
Over 26 weeks on prophylaxis 77% of people had 0 bleeds* and 81% of people had 0 joint bleeds*
*
Data based on treated bleeds.
ABR=annualized bleed rate.
ABR=annualized bleed rate.
Pain and physical health from patients reported outcomes
In people on once-weekly ALTUVIIIO who switched from prior prophylaxis in XTEND-1

Physical health
Change observed in Haem-A-QoL Physical Health Score from 37.0 at baseline to 29.7 at Week 52*

Pain intensity
Reduction observed in PROMIS Pain Intensity 3a Score from 2.5 at baseline to 2.2 at Week 52*
A lower score represents an overall improvement in these measures. The XTEND-1 study was designed so patients and their doctors knew they were taking ALTUVIIIO, which may have impacted these findings. The study was a single-arm study so all participants in the trial were treated with ALTUVIIIO and there was no other treatment to compare, which may impact the ability to assess the effect of ALTUVIIIO on these patient-reported outcomes. The PROMIS tool was not specifically developed for use in hemophilia patients.
A lower score represents an overall improvement in these measures. The XTEND-1 study was designed so patients and their doctors knew they were taking ALTUVIIIO, which may have impacted these findings. The study was a single-arm study so all participants in the trial were treated with ALTUVIIIO and there was no other treatment to compare, which may impact the ability to assess the effect of ALTUVIIIO on these patient-reported outcomes. The PROMIS tool was not specifically developed for use in hemophilia patients.
*
Patient-reported outcomes of pain intensity and physical-health scores were evaluated in patients receiving ALTUVIIIO prophylaxis in Group 1. The PROMIS Pain intensity 3a instrument was used to assess pain, specifically the first question that rates the worst pain experienced during the last 7 days. Physical-health scores were assessed in patients 17 years and older using the Physical Health Score of Haem-A-QoL, which evaluated factors such as painful swellings, presence of joint pain, pain with movement, difficulty walking far, and needing more time to get ready.
Haem-A-QoL=Haemophilia Quality of Life Questionnaire for Adults; PROMIS=Patient-Reported Outcome Measurement Information System.
Haem-A-QoL=Haemophilia Quality of Life Questionnaire for Adults; PROMIS=Patient-Reported Outcome Measurement Information System.
One infusion, a powerful* response
Bleed control with ALTUVIIIO
In the clinical trial of 362 bleeds that occurred
97%
were treated with 1 infusion†
†
In the XTEND-1 study, 350 out of 362 bleeds that occurred were resolved with only one infusion.
*
Based on the number of infusions needed to treat an active bleed.
Find your CoRe Manager and connect today!
Sanofi Hemophilia Community Relations and Education (CoRe) Managers offer education to people living with hemophilia and their families. CoRe Managers provide information about living with hemophilia and treatment options. Use our handy CoRe Locator to find the CoRe team member nearest you.

